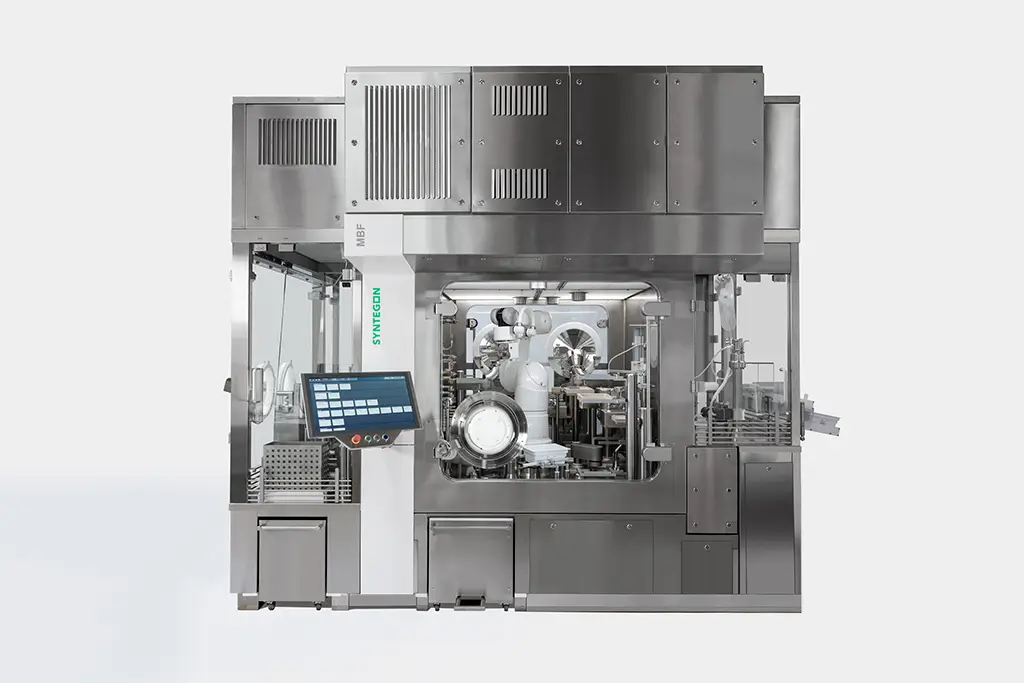
When you’re looking for the right blend of innovative know-how and flexible support for your injectable drug-device product, combine forces with Kindeva. Our investment in a new sterile injectable fill-finish facility in Bridgeton, Missouri, makes us one of only a few CDMOs who can address sterile fill, device manufacture, and final assembly in one geographic location, delivering Annex I compliance with every line. We bring together a ~1M ft2 cGMP footprint, 60+ years of experience, renowned engineers, preclinical to full-scale manufacturing capabilities, and cutting-edge technology to achieve your goals.
Built for the future
Our facility in Bridgeton, MO, is built from the ground up to address today’s most pressing challenges in aseptic injectable fill-finish manufacturing.
- Small-scale clinical, niche commercial to large commercial scale
- Fully integrated capabilities across fill-finish, product assembly, and final product in one geographic location
- Initial capacity to fill 100M+ units across vials, cartridges, & syringes, with infrastructure to support further expansion, impacting millions of patients’ lives worldwide
- 155K+ sq. ft. cGMP footprint
- Facility design principles enable patient safety and Annex I compliance (3 fully isolated high-speed filling lines with utilities for up to 7, automation, unidirectional flow, etc.)
- microBatch isolated filling line which accommodates < 2,500 batch sizes
- DEA Class II-V, controlled substance approval
Sterile filling platforms
Vials/cartridges/syringes
Kindeva offers an unmatched range of development, manufacturing, and aseptic fill-finish solutions for vials, cartridges, and syringes. We can fill any currently available ready-to-use formats, from small-scale single batches to campaign filling, up to 100mL. Our device-agnostic approach assists you in identifying the best fit for your therapy.
Delivering industry-leading automation & efficiency
When it comes to complex and high-cost drugs, we understand that you need every single drop of your product. Through strategic investments, we have achieved a high degree of automation for processes that have traditionally been manual, from PUPSIT to no-touch transfers. This allows us to increase output while reducing errors and improving efficiency. With modular filling suites, we reduce downtime and provide superior scalability.
Injectable delivery platforms
Autoinjectors
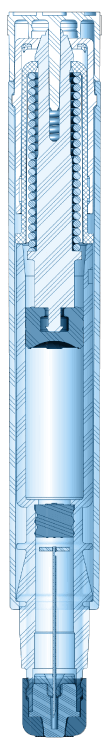
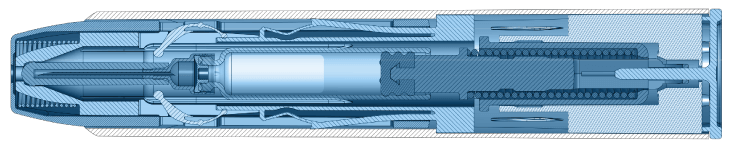
Our patented single- and dual-chamber sterile injection platforms are designed for reliability, ease of use, portability, and durability. As autoinjector innovators for more than 60 years, we leverage an unrivaled breadth of knowledge to ensure every device is of the highest quality and meets FDA combination product guidelines.
Single-Chamber Autoinjector Platforms
- Drug delivered: 0.3mL-3.0mL
- Activation: Two-step user activation/rear safety release/automatic sharp protection
- Status: Launched/on market
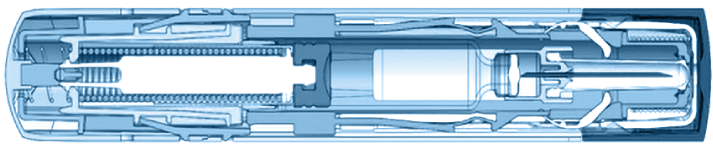
Dual-Chamber Autoinjector Platforms
- Drug chambers: Liquid/liquid
- Drug delivered: 0.7mL-2mL
- Injection: Intra-muscular, drugs separated in vitro and in vivo (enhanced stability and bioavailability)
- Activation: Two-step user activation/rear safety release
- Status: Launched/on market
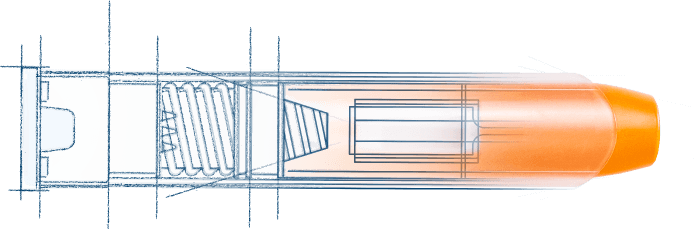
With Kindeva, you and your patients benefit from the latest in autoinjector evolution. Our newest innovations comply with the industry standard two-step sequence of use and accommodate a wide variety of containers and fill volumes in a compact form factor.

Our newest platform at a glance:
- Two designs:
- 0.3mL to 3mL delivery
- <0.5mL delivery
- Intramuscular or subcutaneous injection
- 99.999% minimum system-level reliability
- Meets FDA emergency use guidelines
- Easy adjustability of key user interfaces
- Uncap and activation forces
- Two-step activation
- Remove cap and press and hold down on the injection site
- Built for expansion
- Early designs which incorporate cartridge and dual-chamber options
Support for non-proprietary platforms
We are committed to working with you to ensure optimal delivery of your therapy, whether it is with our platforms or a third-party device. We have extensive experience working with other platforms across all stages of development and manufacturing.
Microneedle-based drug delivery
Kindeva is leading the way in intradermal delivery, with microneedle array patches customized to your needs and specifications. Our systems allow for reliable delivery of vaccines, peptides, proteins, biologics, and small and large molecules, with options for both solid and liquid APIs.
Leverage expert analytical testing
Gain insight into your injectable product’s performance and quality through our analytical and testing services. We deliver unrivaled know-how, backed by over a century of analytical experience and expertise in working with regulatory bodies worldwide. Our services include drug content assays, extractable and leachable identification and characterization, drug content uniformity testing, medical device performance testing, and more.
Combine forces with Kindeva
Kindeva’s injectable technologies provide customized drug-delivery options to suit the needs of your patients or global health security forces — always with therapeutic requirements, reliability, and ease of use at the forefront of each solution.
We can take your product from ideation to commercialization in-house with a continuity of services that enable time and cost savings while ensuring quality.
Your therapy is a force for good. Our expertise brings it into the world.
A Pointed Legacy of Innovation
The Evolution of the Autoinjector
From the very beginning of the autoinjector’s history, Kindeva Drug Delivery has led the way in innovating self-administered injectables. Millions of people worldwide rely on these devices for lifesaving treatment, and we have continually evolved and adapted our platforms to provide a better experience for our patients.
Before the autoinjector
Military personnel and allergy sufferers relied on options that were difficult to use and required a complex series of steps, delaying treatment in emergencies.
1959 Space to the battlefield
One of our first autoinjector platforms was the AtroPen® -providing fast, reliable self-injection options in emergency situations. This platform supplied a number of products to the military as well as NASA, which relied on our autoinjectors starting in 1961 with the Project Mercury spaceflight program onward.
1970 ComboPen® platform
We created this platform to deliver medical countermeasures in the field more efficiently while navigating complex regulations.
2002 BinaJect® platform
With this platform, we brought an industry-leading dual-chamber option to market that enhanced stability for intramuscular injections, keeping drugs separated in vitro and in vivo.
2009 TruJect™ platform
We introduced the next generation of easy and reliable single-chamber autoinjectors with integrated sharps protection.
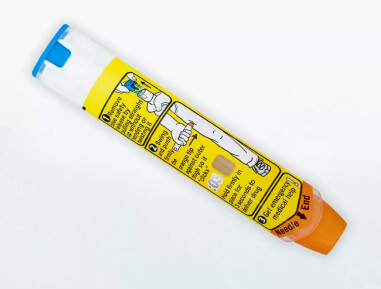
2024 Next-generation autoinjectors
Our next-generation platform is designed for reliability, usability, and patient experience. It is built for expansion with options for single or dual chambers, liquid or dry APIs, and prefilled syringes or cartridges.
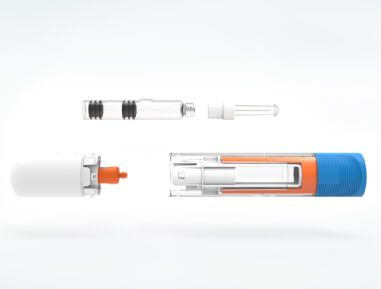
Looking To The Future
With a rich legacy of firsts and a strong foundation of expertise, Kindeva Drug Delivery continues to innovate drug-device combination products. We leverage our century of experience to navigate today’s challenges and bring improved therapies to market across not only injectable but also pulmonary & nasal and transdermal drug delivery.