Combine Forces
Your therapy is a force for good.
Our expertise brings it into the world.
Your leading global combination product CDMO
Kindeva is a global force in drug delivery, offering innovative, comprehensive contract development and manufacturing solutions for partners around the world. From ideation through commercialization, our in-house industry expertise and cutting-edge platform technologies enable flexibility and capabilities you won’t find anywhere else.
Unrivaled experience. Unmatched collaboration.
We bring unparalleled know-how to the device-agnostic development of high-quality drug-device combination products across a wide range of platforms for pulmonary & nasal, injectable, and transdermal therapies.
A Proven Partner
~ 1300 Patents
75 Inventions In Use
~
100
M
Commercial Devices
Shipped Annually
10
Manufacturing and
R&D Facilities
100 + Years of Experience
~ 1 M ft2 cGMP Footprint
2000 + Employees
Join Our Mission
We minimize risks and accelerate timelines by expertly handling everything in-house, all with one ultimate goal: to constantly redefine highest quality with each product we bring to the world. Manufacture more tomorrows for patients alongside us through our new molecule and biologic drug development, comprehensive analytical services, leading sustainability initiatives, and global health security initiatives.
Our Legacy of Innovation
An unrivaled foundation for manufacturing more tomorrows
Since 1956, Kindeva Drug Delivery has actively reshaped the drug-device combination product landscape through a series of unparalleled firsts that established a solid foundation for expanding the future of the pharmaceutical industry.
1956 Invented the pressurized metered‑dose inhaler (pMDI)
Kick-started the evolution of inhaled therapies by developing an easy-to-use device for patients around the globe.
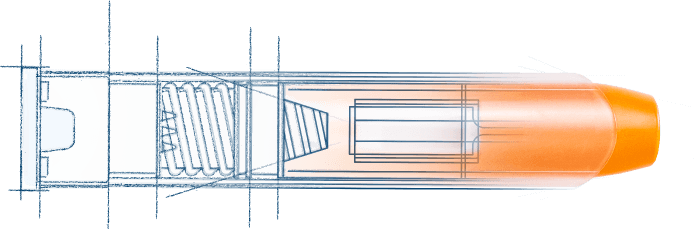
1959 Invented the emergency use autoinjector
Laid the groundwork for the ongoing refinement of self-administered injectables currently in use by millions of people worldwide.
1970 Invented the drug-in adhesive patch
Provided a noninvasive method for delivering a drug over an extended period of time.
1970 Invented the ComboPen® platform
Created to deliver medical countermeasures in the field, this platform informed autoinjector innovations for anaphylaxis.
1989 Invented the breath-actuated inhaler
Offered a lifesaving option for individuals with hand-breath coordination problems that make traditional inhalers difficult to use.
1995 Invented the CFC-free MDI
Set the standard for future care options developed to help safeguard the environment.
1998 Invented the TruJect™ platform
Introduced the next generation of easy and reliable single-chamber autoinjectors, widely used to deliver a variety of medications.
2002 Invented the BinaJect® platform
Brought an industry-leading dual-chamber option to market that enhanced stability and bioavailability.
2010 Developed the first commercially available dose counter
Ensured patients knew the number of inhaler actuations remaining so they could always be prepared.
2012 Invented the CFC-free nasal MDI
Crafted a more environmentally sound device for drug delivery capable of bypassing the blood-brain barrier.
2023 Developing microneedle-based drug delivery platforms
Leading the charge creating accurate, reliable intradermal delivery with the potential to eliminate cold-chain storage and enhance immunogenicity and efficacy.
2025 Developing low-GWP inhalers
Planned opening of one of the first commercial green propellant lines for filling inhalers using propellants with up to 99.9% lower Global Warming Potential than current options.
Continuous Innovation
Kindeva has fueled the advancement of the drug-device combination product industry from the start, and we use this expansive know-how to bring better products to market for our partners and their patients every day.
Your product is a force for good. Our expertise brings it into the world.